Bioélectrochimie, Biointerfaces, Biotechnologies
 Bioélectrochimie, Biointerfaces, Biotechnologies
Bioélectrochimie, Biointerfaces, Biotechnologies
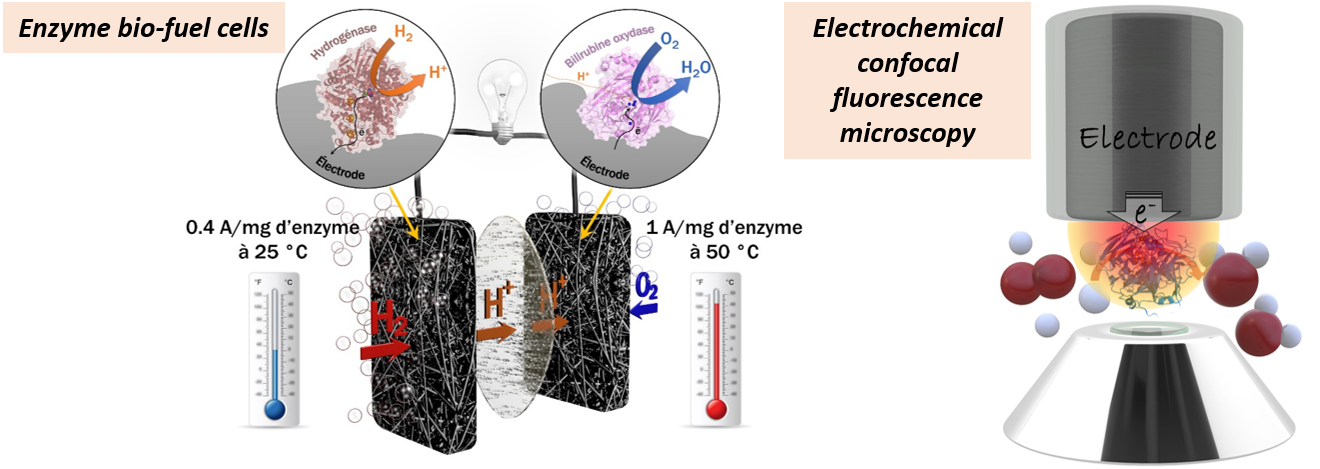
Electron transfers in energy metabolic chains occur through transitory protein complexes. Our main objective is to understand and control the molecular basis that drive efficient electron transfers in order to use the proteins involved in bioelectrocatalysis.
To do that, we mimic physiological electron transfer pathways by replacing the physiological partner of the protein/enzyme by an electrode. This electrode is chemically functionalized to ensure stable enzyme immobilization and favour an efficient electron transfer.
We consider both planar and nanostructured electrodes (carbon nanotubes, metal nanoparticles, transparent porous electrodes). We use methods of numerical simulation to model enzymatic reactions and mass transport in the porous bioelectrodes in order to optimise their design.
To get a deeper insight in enzyme immobilization and in fine to find designs increasing the bioelectrode efficiency and stability, we develop the coupling of physical methods with electrochemistry (e-SPR, e-QCM, e-fluorescent confocal microscopy).
In terms of applications, our research especially focuses on developing enzymatic biofuel cells and enzymatic biosensors.
Responsable de l’équipe : Elisabeth LOJOU
Site web équipe : https://bip.cnrs.fr/groups/bip08/research/
Correspondant GDR : Anne de Poulpiquet
Site web labo : https://bip.cnrs.fr/
Mots-clés
Redox enzymes
Electrocatalysis
electrode structuration
numerical modeling; In situ and in operando methods